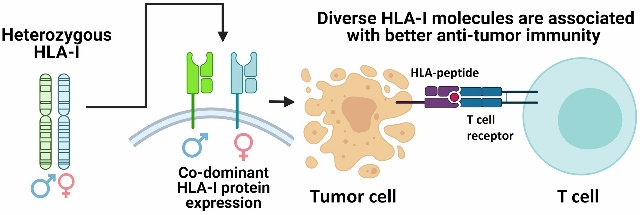
What if someone tells you that possessing diverse genes could improve your chances of combating infections and even fighting off cancer? The divergent allele advantage hypothesis tries to explain this phenomenon. It states that possessing a greater variety of certain genes (alleles are variants of a gene) can improve individual survival. That would be just another derivation of the phrase “strength lies in differences, not in similarity,” but applied in the context of biology. Researchers found support for this hypothesis in the case of individuals with a rich diversity of human leukocyte antigen (HLA) complex proteins. HLA protein diversity has been extensively studied to date, and has been found to directly influence successful immunologic control of both infectious disease and cancer.
Functionally, HLA proteins are involved in immunological surveillance, where they help the immune system recognise ‘self’ (healthy cells of an individual) from ‘non-self’ (foreign cells). HLA proteins come in various forms, with variations located in the peptide-binding region. Each type of HLA-protein can help the immune system recognise a limited set of non-self protein-fragments (also known as peptides) produced by an individual cell. This means that having a wide variety of HLA proteins allows an individual’s immune system to recognise a broad range of non-self peptides that are produced by invaders like bacteria, viruses, and parasites. T-cells (a type of white blood cell that generates anti-tumor responses) recognise HLA-peptide signals, become activated, and initiate immune responses against the peptide-producing cells. Different T-cells recognise specific HLA-peptide signals. As a result, the interaction between diverse HLA proteins and T-cells leads to increased immune surveillance against non-self peptides, since having a wider variety of HLA proteins allows the immune system to respond to a broader range of pathogens, thus improving individual survival. Intriguingly, cancer cells undergoing frequent mutations often also generate peptides that can be recognised as foreign material by the immune system, resulting in anti-cancer immune responses.

Recently, researchers tested how divergent HLA allele advantage can play an important role in anti-cancer immunity, and how it can dictate therapeutic outcomes for cancer treatment. Diego Chowell and colleagues studied cancer patients undergoing a novel form of therapy called checkpoint blockade immunotherapy. This antibody-based therapy restores the functionality of the immune system by removing ‘molecular brakes’ on T-cells. These ‘brakes’ are expressed by cancer cells in the form of immune checkpoint proteins, which inactivate T-cells that would otherwise destroy the cancer cells. In the presence of checkpoint-blocker antibodies, the immune checkpoint proteins can no longer suppress T-cell activity, resulting in strong anti-tumor responses. The researchers hypothesized that since this therapy is based on anti-cancer T-cell activity, the therapeutic outcomes may also depend on the HLA-diversity of an individual patient. Because a patient with higher HLA-diversity can activate a greater variety of T-cells, those patients would likely benefit more from the therapy, since their immune systems would be more likely to be able to respond to the cancerous cells. The researchers therefore predicted that HLA-diversity would predict survival after treatment with checkpoint blockade therapy.
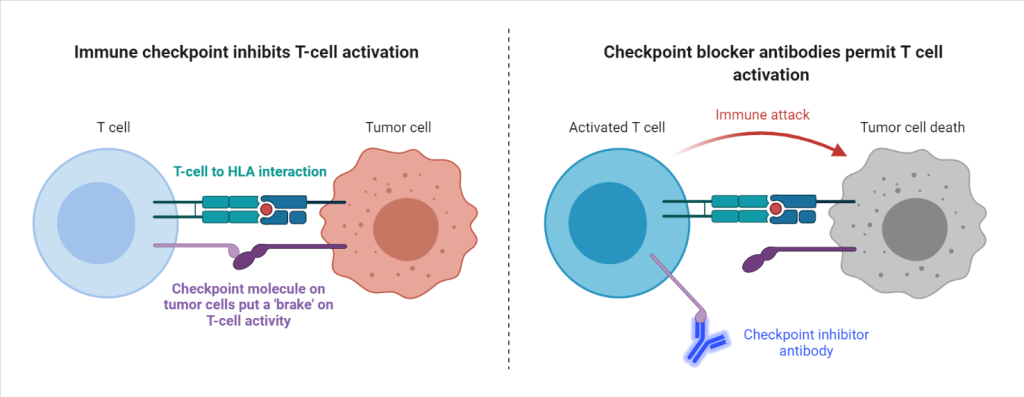
To test their prediction, Chowell and colleagues quantified the diversity of HLA genes of each patient and examined the association with therapeutic success after checkpoint-immunotherapy.
In the researchers’ preliminary study, they determined the HLA genotype of 1535 advanced cancer patients treated with checkpoint-immunotherapy. Patients with a higher diversity at HLA-genes (having different gene variants, also known as heterozygous) had improved overall survival after checkpoint-immunotherapy when compared to patients who were homozygous (had the same gene variants) for at least one HLA variant. This is because individuals with heterozygous HLA-variants can present up to twice as many peptides to T-cells as compared to homozygous individuals, supporting the divergent allele advantage hypothesis. Also consistent with the hypothesis, the researchers found that patients that were previously heterozygous at HLA, but became homozygous due to mutations acquired during their lifetime (known as somatic loss of heterozygosity) were also associated with poor outcome.
In a later study, Chowell and colleagues demonstrated how among fully HLA heterozygous patients, those with a higher HLA sequence-diversity responded better to checkpoint-immunotherapy than patients with a lower HLA sequence-diversity. The researchers quantified HLA sequence-diversity as HLA evolutionary divergence (HED), which is indicative of sequence variety and structural diversity of HLA proteins among individuals. Moreover, HED strongly impacted the diversity of peptides (tumor, viral and self-peptides) that could be presented to T-cells.
These results suggest that HLA genotypes and loss of diversity in HLA genes can influence cancer patient survival, and should be considered as potential screening factors for trial-design and treatment with checkpoint-immunotherapy. Additionally, Chowell and colleagues’ work emphasizes the importance of considering individual diversity towards disease resistance, because these differences can explain why patients respond differently to the same treatment, can help in estimation of disease susceptibility and recurrence, and allow researchers and physicians to ‘match’ an individual to the most promising therapy available. Therefore, evolutionary concepts such as the divergent allele advantage hypothesis can have implications for immune recognition and for the design of antigens for cancer vaccines and immunotherapies. Moreover, such studies emphasize the importance of an evolutionary framework to solve challenges in medicine and health care.
Articles discussed
- Chowell D, Morris LGT, Grigg CM, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018 Feb 2;359(6375):582-587. doi: 10.1126/science.aao4572.
- Chowell, D., Krishna, C., Pierini, F. et al. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat Med 25, 1715–1720 (2019). https://doi.org/10.1038/s41591-019-0639-4
Illustrations: Designed in Bio-Render.
Darshak Bhatt is a PhD student at the department of Experimental Oncology at the University of São Paulo and at the University Medical Center of Groningen. You can find him at his blog or on twitter @DarshakWrites.

One thought on “Embracing differences to boost anti-tumor immunity: How HLA diversity can improve cancer therapy”